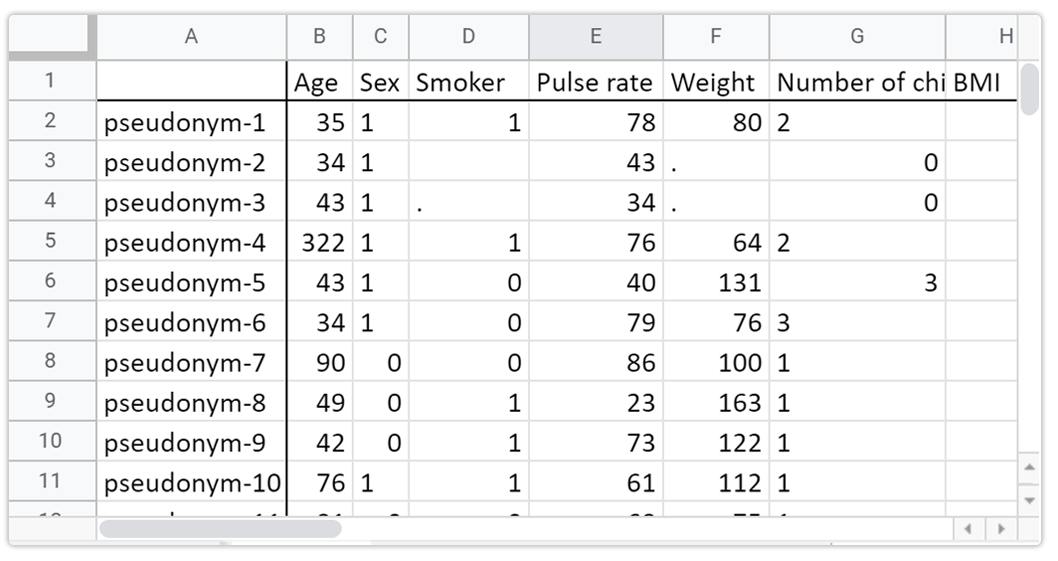


It's like Excel for Clinical Research
We replace the Excel database you are using in your clinical research, instantly enabling collaboration, GDPR compliance, and simplifying your data management.

“Sensivo gives us the same
workflow as Excel, and adds real-time collaboration, user roles and GDPR compliance to our clinical studies.”


FAQ
Can I upload personal/sensitive data to Sensivo?
Yes, Sensivo is GDPR compliant and has servers in Stockholm, Sweden provided by ' SafeSpring '. We also will send you a DPA on request. Please Contact Us for more information.

How much does it cost?
Sensivo is free to use. We also have a premium and on-premise solution, please take a look at Pricing page for more information

Why Sensivo is better than Excel?
Sensivo also uses a spreadsheet interface but adds a GDPR layer on top of it and special features tailored for clinical research. Such as pseudonymization, user management, and so on.

“Sensivo is easy to use, and with the spreadsheet interface and user-friendly design, we have a better insight of our data.”



